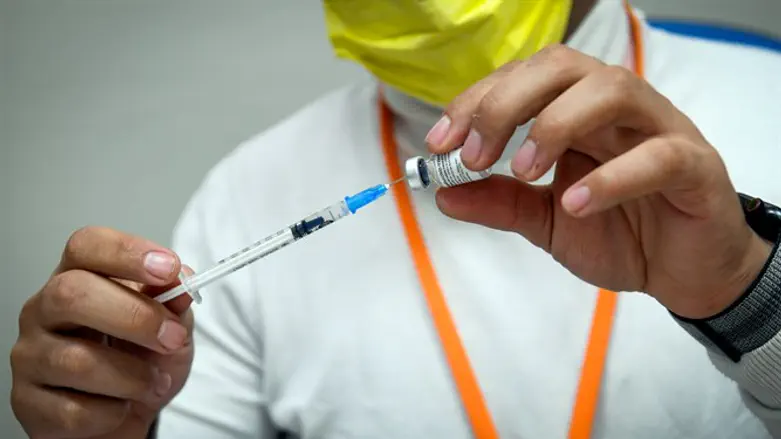
The European Medicines Agency, the medical watchdog of the European Union, announced on Monday that it has approved the application of coronavirus booster shots for people who have received two doses of the coronavirus vaccine.
The approval applies to people over the age of 18 who received the second dose of the Pfizer or Moderna coronavirus vaccine at least six months prior. People with weakened or compromised immune systems would only have to wait 28 days after their second shot.
Last month, the US Food and Drug Administration authorized booster doses of COVID-19 vaccines for Americans aged 65 and older, younger adults with underlying health conditions and those in jobs that put them at high risk for COVID-19. The FDA did not authorize booster shots for healthy adults.
