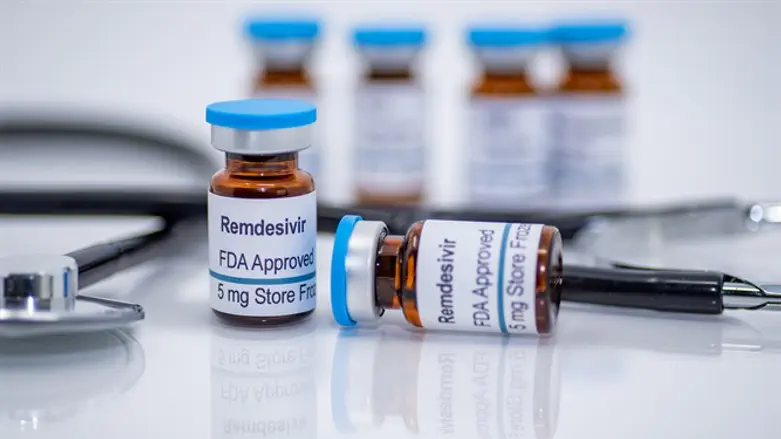
The US Food and Drug Administration on Thursday approved Gilead Sciences Inc’s antiviral drug remdesivir for treating patients hospitalized with COVID-19, making it the first and only drug approved for the disease, Reuters reports.
Gilead said it is currently meeting real-time demand for remdesivir, which is sold under the brand name Veklury, in the United States and anticipates meeting global demand by the end of October.
Remdesivir, previously granted emergency use authorization by the FDA for COVID-19, was one of the drugs used to treat US President Donald Trump’s recent coronavirus infection.
The European Commission has also authorized the use of remdesivir to treat the new coronavirus.
At least two major US studies have shown that remdesivir can reduce the duration of hospital stays for COVID-19 patients, but doctors have remained wary of using the drug in patients with less severe illness.
The World Health Organization last week said its international trial of COVID-19 therapies found that remdesivir did not have a substantial effect on patients’ length of hospital stay or chances of survival. The study has not been reviewed by outside experts.
