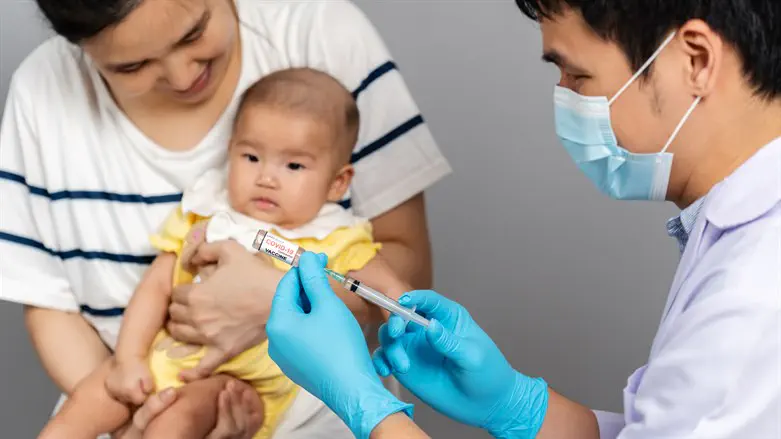
Health Ministry Director General Professor Nachman Ash has approved the administration of Moderna's Pfizer-BioNTech's COVID-19 vaccines for infants and children ages six months to five years.
Though the approval applies to all children in the above age group, the Health Ministry has not issued a general recommendation for the vaccine.
The vaccines were approved last month by the US Food and Drug Administration (FDA), and later the same month by the Israeli Health Ministry's Staff for the Management of Pandemics.
The Health Ministry has already begun the process of purchasing COVID-19 vaccines for young children from Pfizer-BioNTech, Kan 11 reported last month.
According to the FDA, the littlest children developed high levels of virus-fighting antibodies, comparable to what is seen in young adults, the FDA said. Moderna's vaccine was about 40% to 50% effective at preventing infections but there were too few cases during Pfizer's study to give a reliable, exact estimate of effectiveness, the agency said.

