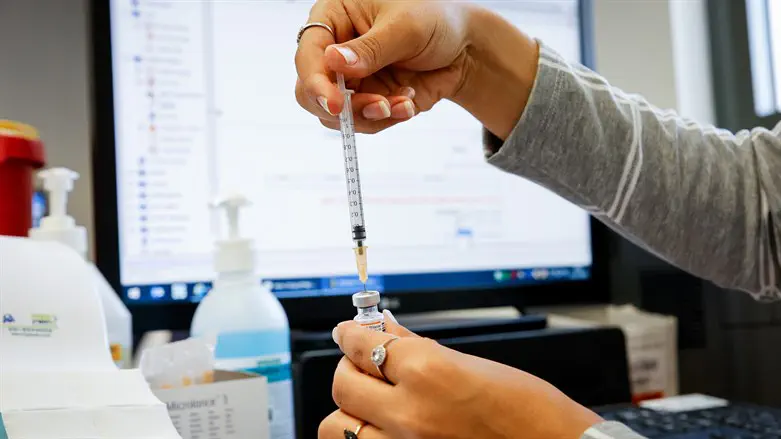
Preliminary results from the first study of its kind in the world, led by Sheba Medical Center, on the safety and efficacy of the fourth dose of the coronavirus vaccine, indicate a significant increase in antibody levels, but this level of protection is only partially effective against the Omicron variant.
The study, led by Prof. Gili Regev-Yohai, director of the Infection Prevention and Control Unit at Sheba Medical Center, examined the rate of increase in antibodies after the fourth vaccine of both the Pfizer and Moderna vaccines was administered, compared to a control group.
The objectives were to test the efficacy and safety of vaccines and whether the combination of vaccines from different companies would affect the rate of increase in antibodies.
Preliminary results indicate that when the Moderna vaccine was given to a person who had received three doses of the Pfizer vaccine, the resulting increase in antibodies is similar to when the fourth dose is the Pfizer vaccine after one week. In addition, the Pfizer vaccine presented a further increase in antibody levels after two weeks as opposed to just one week.
The Pfizer and Moderna vaccines were found to have similar levels of safety.
Prof. Gili Regev-Yohai explained that "the increase observed in the level of antibodies both after the administration of the fourth dose of Moderna and after the administration of the fourth dose of Pfizer is slightly higher than the peak level observed after the administration of the booster dose."
"Given the exclusive data from Sheba Medical Center on the Omicron disease among Sheba employees participating in the large serology study, we understand that despite the significant increase in antibodies after the fourth vaccine, this protection is only partially effective against the Omicron strain, which is relatively resistant to the vaccine."
